A connected factory is the solution to increasing complexity in pharma production.
NNIT, explains why pharma companies need fully integrated systems to keep up with increasing demands and advanced new treatments.
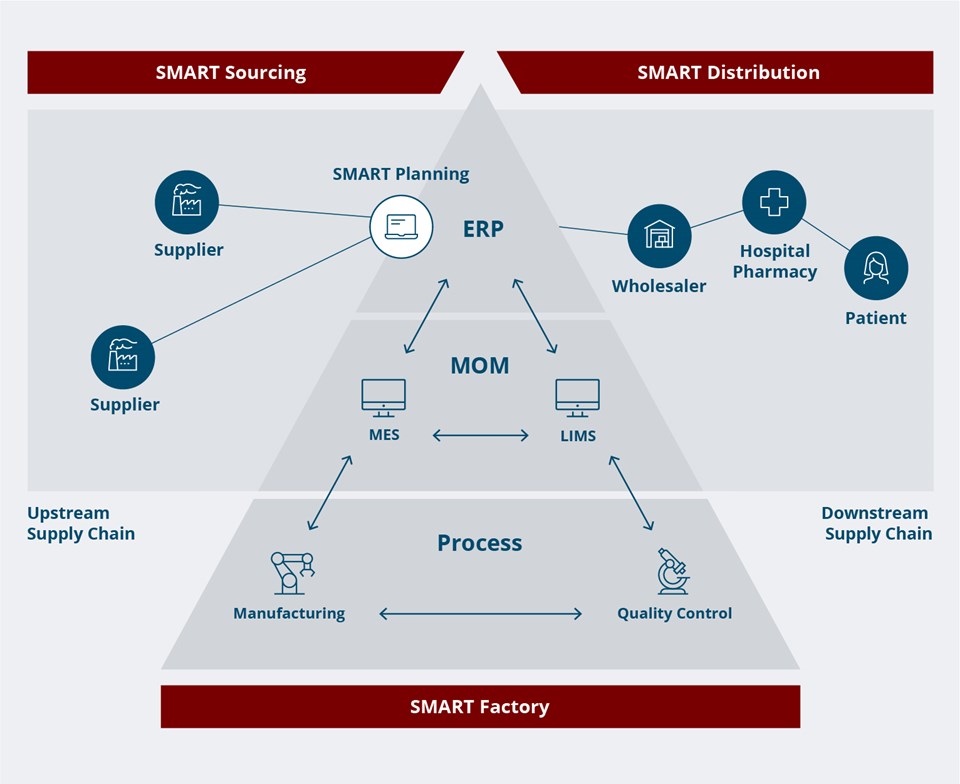
A modern pharma production site has numerous layers of interconnected processes and dependencies, both within the site itself and throughout the supply chain.
And while pharma production has never been simple, multiple factors and industry trends are rapidly increasing the level of complexity to a point that is impossible to manage without a fully integrated factory.
The systems used in pharma production have to match the complex reality:
– The pharma factory of the future requires full integration, both within the factory from MES to the operators and production equipment, but also up to the ERP and horizontally along the upstream and downstream supply chain. This is not just a technology challenge. To achieve the factory of the future, you will also need to focus on business strategy, regulatory compliance and change management.
Advanced treatments are more complex
One of the contributing factors to the increase in complexity is the development of new advanced therapies, such as biotech, stem cells, and cell and gene therapy. Biopharmaceutical components such as large-molecule proteins and antibody-like biomolecules present vastly different challenges than chemical compounds.
– The common denominator for biotech products is that they are more complicated to manage compared to traditional pharma products, both in the supply chain and production execution.
Demand for personalized medicine
The advances in treatments and greater insight into how different individuals respond to them has also created a rising demand for specialized medicine, which target specific populations and sub-categories of patients. The ultimate end-goal is the ability to treat each individual patient with products tailored to their unique needs, medical history, and genetic profile.
– The movement towards personalized medicine requires a dynamic production environment capable of producing micro-batches on demand. This is impossible to do without a fully integrated production and supply chain, which is able to adjust product recipes to match patient data and ensure that the finished product is registered, serialized, and delivered to the right location.
M&A add complexity
Organic growth can be difficult to manage in itself, but mergers and acquisitions add a whole new layer of complexity to pharma production. When two companies merge into one, both IT and the rest of the business often struggle with the challenges of unify diverging IT/OT strategies, infrastructures, non-harmonized master data, and system standards.
– When a pharma company acquires an existing production site, there are many complex decisions to be made, such as whether to replace the existing ERP system or update the platform that collects data from the production lines. In many cases, these existing systems have functionality and dependencies which is critical for managing production.
Pharma in the Cloud
Adoption of cloud computing is increasing across virtually any sector and the life sciences industry is no exception. Pharma companies are eager to unlock the potential of cloud-based alternatives to on-site systems like MES.
– Today, only about five percent of pharma companies operate cloud-based MES, but that number is likely to grow fast. The switch is coming, and we see multiple vendors launching cloud-based platform solutions. Managed cloud solutions reduce the pain of cybersecurity, which is another cost intensive area. But before migrating to the cloud, pharma companies must first consider the complex implications for their legacy IT, existing production equipment, cybersecurity, and regulatory compliance.
Need help navigating the complexities of integrated pharma production?
The complex reality of modern pharma production increases the need for an end-to-end partner who can see the big picture, including technical expertise, manufacturing processes and regulatory guidance.
At NNIT, we can assist you with both business consulting, system implementation and operation, allowing you to make the best choices for strategy, technology and change management.
Do you want to know more?
Download our whitepaper "The Pharma Factory of the Future is Fully Integrated":
The whitepaper delves into challenges and the potential benefits of an integrated factory, best practices and solutions as well as common mistakes and pitfalls.