A full, accurate and updated overview of all your manufacturing and supply chain processes enables the life sciences operations business to streamline manufacturing processes, prevent failed batches and time-consuming rework, and to release finished products faster.
If you want to optimize your life sciences manufacturing and supply chain processes, you need up-to-date and accurate information. Relevant data about every step in your value chain should be available to the right people at the right time: from production managers planning and checking the status of manufacturing batches in the shop floor and warehouse, to production supervisors verifying the availability of ingredients and equipment, operators monitoring the critical parameters, and logistics partners picking up the finished product.
Being able to instantly act on that information increases efficiency and prevents recalls and failures. It means you can make corrections in real time, rather than having to scrap whole batches because potential problems were not caught until after the process was complete. You can react to immediate trending and warnings, such as the use of wrong raw materials or deviations from the correct process parameter levels. And you can reduce the time and cost of having finished products stuck in quarantine, waiting to be released by quality control.
Adapt your processes to a connected reality.
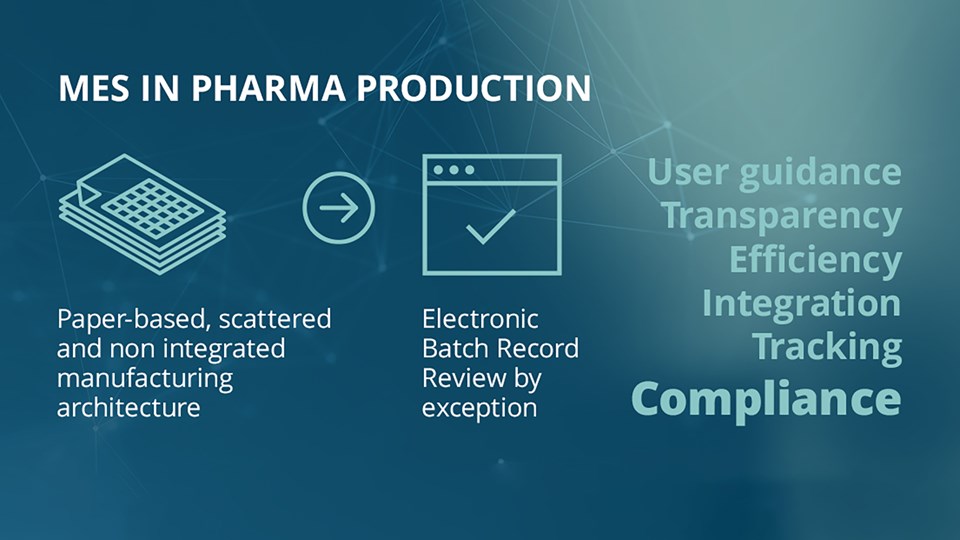
To achieve optimized manufacturing and supply chains processes, you must replace traditional isolated and paper-based ways of working with tightly integrated and validated digital tools. The old ways are no longer sufficient to handle the increasingly complex landscape of modern pharma production, where new production methods, increasing regulatory demands and shorter delivery cycles are constantly pushing the envelope.
Optimizing and digitalizing processes is not just a matter of upgrading software or implementing new systems such as a manufacturing/laboratory execution system (MES/LES) or information management system (MIS/LIMS). Your old processes were designed to match your old tools and isolated information flows. If you implement digital tools without implementing related features into your processes, you're leaving efficiency on the floor.
Furthermore, the nature of digital tools in life sciences companies has changed over the past decade. Today, focus is more on data and content management, like setting up workflows, running data analytics and information visualization, and less on technical details such as installation and software customizations. This requires a different approach to validation, master data management and process mapping.