Integrated production is the steppingstone to the fully digitized pharma factory
Integrated systems and consistent use of data standards are the next steps towards the Pharma Factory of the Future.
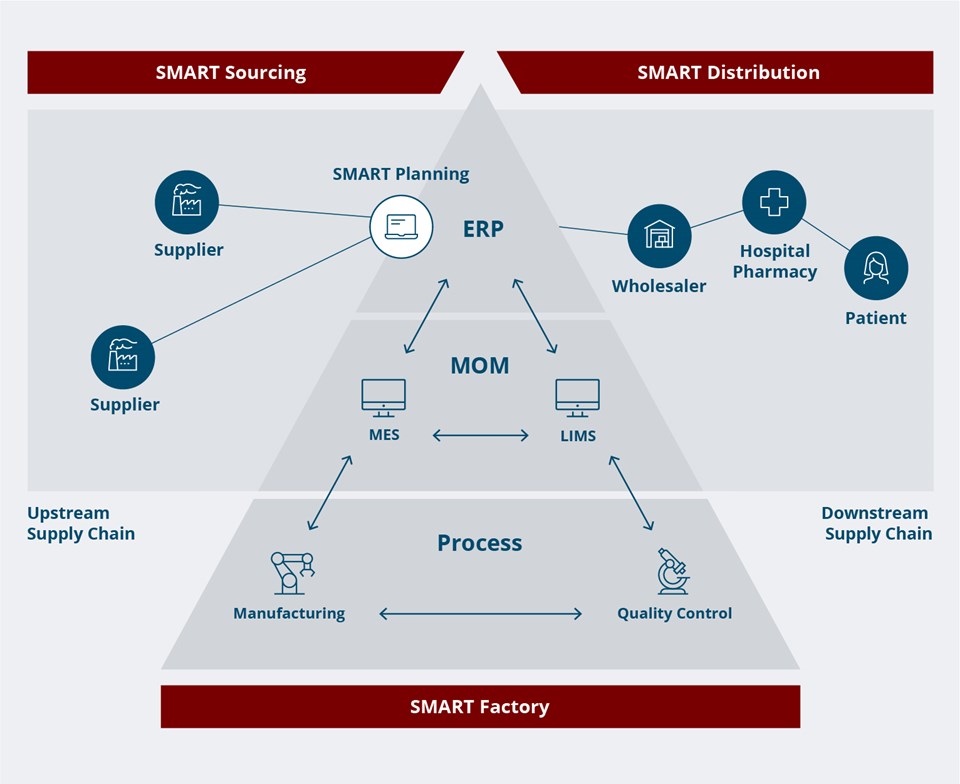
Better horizontal and vertical integration is essential for reducing the need for manual data entry. It is all about trustworthy and reliable data, which will enable advances like digital twins, AI-driven optimization and fully integrated production.
Reducing manual interventions and records is a critical element in managing the increasing complexity of pharma production. Integrated production enables the development of advanced treatments such as personalized medicine, which is far too complex to manage manually.
For many life sciences companies, the ultimate goal is attaining a fully integrated production while remaining compliant with GxP regulation. This means that pharma companies must ensure that they have the necessary integrations in place to enable a reliable flow of data both vertically in their production and horizontally throughout their supply chain.
To facilitate the full digitalization of pharma production, it is essential to systematically eliminate manual data entry and replace it with integrated data directly from the process equipment.
We often see that manual data is inconsistent, can be contested and when merged with integrated data, it corrupts the integrity of the full stream of data, says Paul Clarke, CTO at SL Controls Ltd., an NNIT Group company.
Guidance for operators
If manual records cannot be completely eliminated as part of the initial migration phase, they can be framed to guide the operator via digitalization. This reduces the risk of errors and increases the overall quality of the data.
– Examples include directing the operator to specific locations to perform verification, instructing the operator on procedures via user interaction with apps and providing predefined drop-down responses to better structure the input. Such measures can achieve significant results and time savings, says Paul Clarke.
No natural data flow
Unfortunately, in the current pharma production landscape, data flows between the various levels within the factory rarely occur naturally. Typically, pharma production IT/OT solutions only deliver data related to their specific functions.
This means that any functions required by systems in other levels, for example the MES, will need to be connected to the lower levels through existing systems. This also applies to horizontal integrations across different equipment or to external sources.
– We often experience failures in solutions that rely on secondary data sources or third-party solutions. In other cases, we see that higher levels in an organization attempt to utilize summarized data sources that simply do not exist. You cannot take for granted that your systems are able to communicate and exchange data in the manner and format needed, Paul Clarke explains.